
Copper is an important metal material because of its excellent thermal, mechanical, and electrical properties, and it is widely used in industry, agriculture, construction and daily use. Copper would lose its beautiful appearance and its excellent thermal, electrical, and mechanical properties if being exposed to moist and chlorine ion-rich environment. In order to improve the service life of copper, surface protection is needed for copper.
Some organic compounds with O, N, and S elements can form coordination bonds or covalent bonds with copper and are widely used as copper corrosion inhibitor. Epoxy functionalized silica sol-gel is widely used in the protection of magnesium, aluminum, and iron alloys because of its water solubility, high stability and reactivity of organic functional groups. However, epoxy functionalized silica sol-gel coating does not provide a good anticorrosion performance when used on copper, because Si-OH has difficulty forming a stable Cu-O-Si bond on the copper surface.
To find the solution, researchers from the Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences (NIMTE) have done a long-term research and recently, made progress on epoxy functionalized silica sol (ESol) and thiourea (TUA).
Thiourea is added to epoxy functionalized silica sol-gel (ESol) coating to improve the adhesion between the coating and copper surface and enhance the physical barrier effect due to the denser coating compared to the regular ESol coating.
Electrochemical methods and salt spray test results revealed that ESol+TUA coating shows the best anticorrosion ability. The corrosion current densities are 9.96×10-8, 1.76×10-8, and 4×10-9 A cm-2, respectively, for ESol, ESol+DETA, and ESol+TUA samples, and the corrosion current density of bare copper is 7.01×10-7 A cm-2. The coating significantly reduces the corrosion current density of the anode and cathode. The ESol+TUA coating shows a very good resistance to salt spray, and the rust only appears at the edge of the copper after being exposed in salt spray for 178 h.
The approach is simple, convenient and easily prepared, which the researchers believe, could be taken into use and would show a bright future. Related results were published on the journal of Surface and Coating technology, 213 (2012) 175-192.
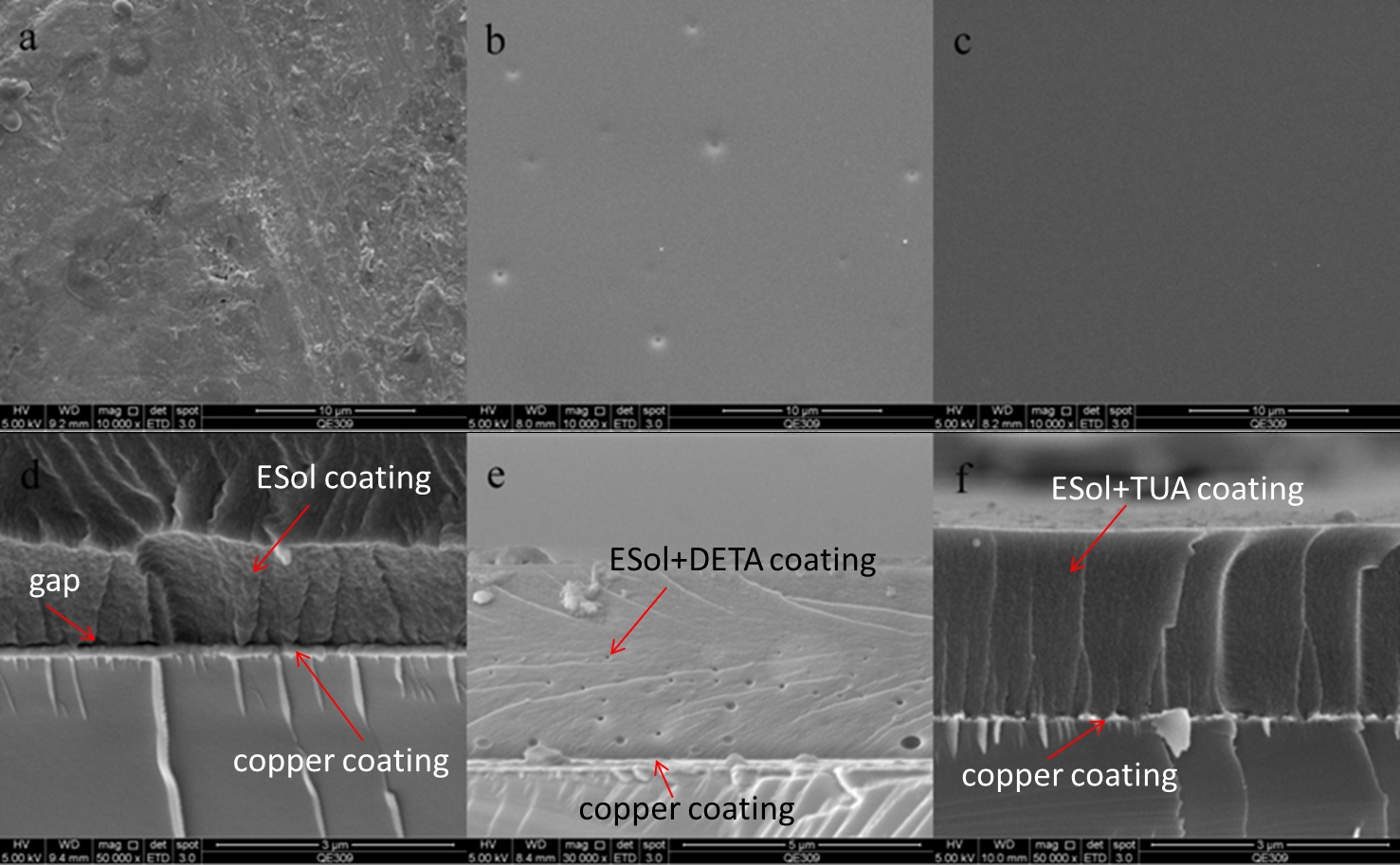 |
|
Fig.1. Surface and cross section morphologies of sol-gel coating covered samples: (a, d) ESol; (b, e) ESol+DETA; (c, f) ESol+TUA. (Image by NIMTE) |
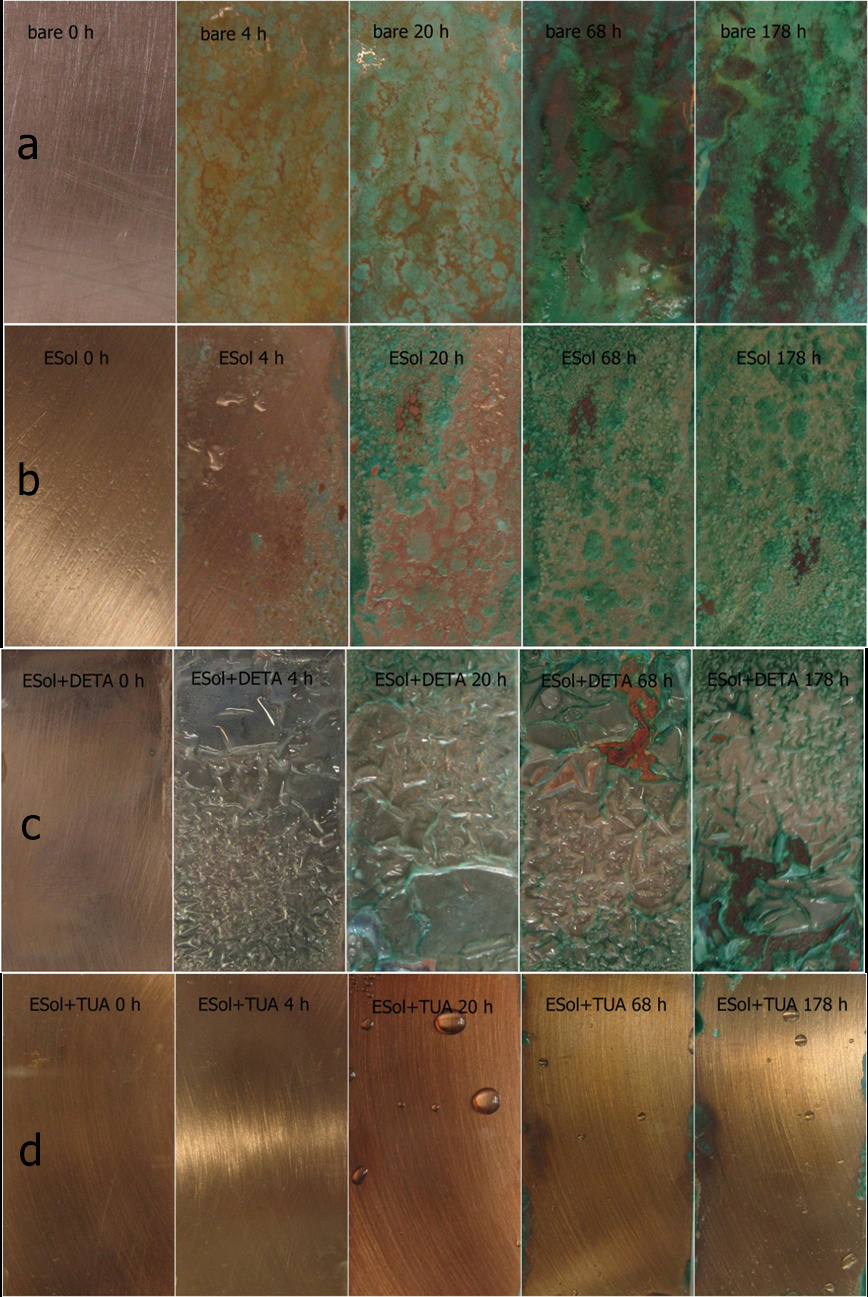 |
| Fig. 2. Photographs of salt spray test after different exposure times (0 h, 4 h, 20 h, 68 h and 178 h): (a) bare copper, (b) ESol, (c) ESol+DETA, and (d) ESol+TUA coated copper substrates (Image by NIMTE) |
CONTACT:
Professor WU Xuedong: xdwu@nimte.ac.cn
Research Staff url: http://english.nimte.cas.cn/pe/fas/200909/t20090929_44484.html

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

